Kinetic Energy and Molecular Speeds
Kinetic Energy and Molecular Speeds: Overview
This Topic covers sub-topics such as Average Kinetic Energy of Gas
Important Questions on Kinetic Energy and Molecular Speeds
By what factor does the average velocity of a gaseous molecule increase when the temperature (in Kelvin) is doubled ?
If a gas expands at constant temperature, it indicated that:
Which of the following expressions correctly represents the relationship between the average molar kinetic energy molecules at the same temperature ?
The root mean square speeds at STP for the gases are in the order:
Give one word for the following. Measure of kinetic energy possessed by the gas molecules.
At any particular time different particles in the gas have __I___ speed and hence _II__ kinetic energies.Here, I and II refer to
In kinetic molecular theory, it is assumed that the average kinetic energy of the gas molecules is directly proportional to the . Here, refers to:
The different molecules possess different velocities and hence different _____.
The ratio of most probable velocity to the average velocity is
By what factor does the average velocity of a gaseous molecule increase when the absolute temperature is doubled ?
Which one of the following statement is NOT true about the effect of an increase in temperature on the distribution of molecular speeds in a gas?
As the temperature is raised from 20°C to 40°C, the average kinetic energy of neon atoms changes by a factor of which of the following ?
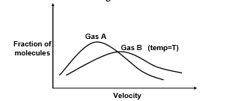
Which of the following statement is incorrect for the given graph?
A thin glass tube closed at one end and open at the other contains a column of dry air which is trapped by a column of mercury as shown in the figure.
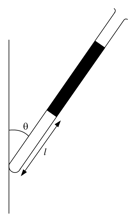
Which one of the following diagrams shows the variation of the length of the air column with the angle of the tube to the vertical?
Two flasks and have equal volume. is maintained at and at .While contains gas, has an equal mass of gas. Assuming ideal behaviours for both the gases, answer the following:
Flask in which the total kinetic energy is greater
Which of the following has the maximum value of mean free path ?
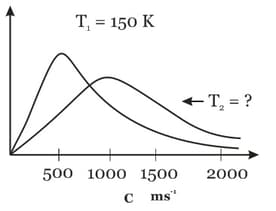
The diagram shows the Maxwell's speed distribution curves for a certain ideal gas at two different temperature T1 and T2. After calculating T2, determine the rms speed of gas at 400 K.
The root mean square velocity of one mole of a monoatomic gas having molar mass M is Urms. The relation between the average kinetic energy (E) of the gas and Urms is
As the temperature is raised from to , the average kinetic energy of neon atoms changes by a factor of which of the following?
The root-mean-square velocity at for the gases , and are in the order